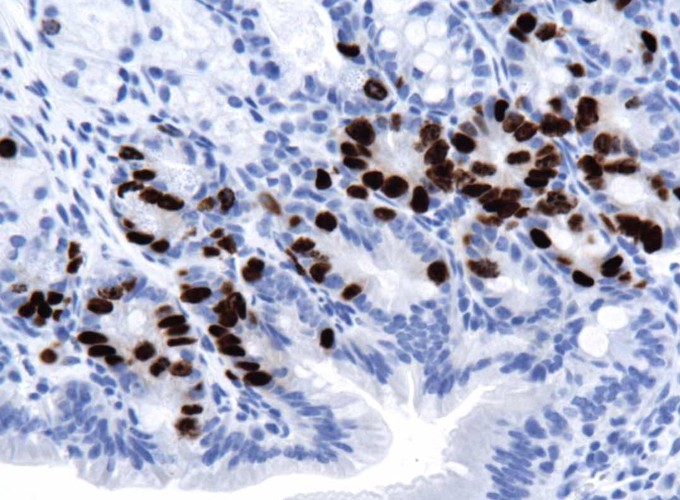
Antibody - Dako mouse monoclonal clone Bu20, cat# M0744 1. Heat tissues to 60°C for 10 mins 2. Deparaffinize and rehydrate tissue sections through series of xylenes and ethanol incubations a. Xylenes or alternative (Citrisolve, Histoclear) incubate 2 times, 5 min each incubation (2 x 5 min) b. 100% ethanol, 5 min c. 95% ethanol, 5 min d. 70% ethanol, 5 min 3. Rinse the slides once in dH20 4. Wash with PBS for 5 mins 5. Antigen retrieval: Preheat BD retrievagen A solution (BD Pharmigen cat# 550524) in coplin jar in steamer. Add slides to coplin jar and incubate for 10 min in steamer then let stand at room temperature for 20 min. 6. Wash PBS 2 x 5 min 7. Antigen retrieval: warm Proteinase K (200µg/ml ) solution to 37°C. Incubate slides for 1 min at RT with proteinase K solution. 8. Block: Quench endogenous peroxidase using 3% H202 in methanol for 20 mins @RT 9. Block: incubate in PBS/5% BSA for 15 min at RT. 10. Primary antibody: Dilute anti-BRDU mouse monoclonal 1:100 in PBS/2.5% goat serum,/0.05% Tween20 overnight at 4°C. 11. Wash in PBS 3 x 5 min 12. Block: biotin block using Vector kit (cat# SP-2001) 13. Wash PBS 2 x 5 min 14. Secondary antibody: dilute anti-mouse biotin (Molecular Probes B-2763) 1:100 in PBS and incubate for 1HR at room temperature 15. Make ABC solution (Vector Vectactain ABC Elite PK-6100) 16. Wash PBS 2 x 5 min 17. Incubate in ABC solution for 20 min at room temperature 18. Wash PBS 2 x 5 min 19. Develop: use DAB or other peroxidase substrate
BRD-U Immunohistochemistry on Mouse - Paraffin